Table Of Content
QuestionPro’s powerful features, such as random assignment of participants, survey branching, and data visualization, enable researchers to efficiently conduct and analyze quasi-experimental studies. The platform provides a user-friendly interface and robust reporting capabilities, empowering researchers to gather data, explore relationships, and draw meaningful conclusions. For instance, providing public healthcare to one group and withholding it to another in research is unethical. A quasi-experimental design would examine the relationship between these two groups to avoid physical danger.
Quasi-experimental Designs That Use a Control Group but No Pretest
How do I design research for an experimental research design in the marketing and tourism field? - ResearchGate
How do I design research for an experimental research design in the marketing and tourism field?.
Posted: Thu, 01 Dec 2022 08:00:00 GMT [source]
Because the quasi-experimental study design has recognized limitations, we sought to determine whether authors acknowledged the potential limitations of this design. Examples of acknowledgment included mention of lack of randomization, the potential for regression to the mean, the presence of temporal confounders and the mention of another design that would have more internal validity. Since quasi-experimental designs are used when randomization is impractical and/or unethical, they are typically easier to set up than true experimental designs, which require[9] random assignment of subjects. Also, this experimentation method is efficient in longitudinal research that involves longer time periods which can be followed up in different environments. Quasi-experimental designs are useful in situations where randomized controlled trials are not feasible or ethical. They provide researchers with an alternative method to evaluate the effectiveness of interventions, policies, and programs in real-life settings.
Example Comparing A True Experiment And Quasi-Experiment
Quasi-experimental research designs find applications in various fields, ranging from education to public health and beyond. One significant advantage of quasi-experiments is their feasibility in real-world settings where randomization is not always possible or ethical. Natural experiments take advantage of naturally occurring events or circumstances that mimic the random assignment found in true experiments. Participants are exposed to different conditions in situations identified by researchers without any manipulation from them. Table 2 provides examples of studies using SWD that have used one or more of the design approaches described above to improve the internal validity of the study. In the study by Killam et al 2010 (31), a non-randomized SWD was used to evaluate a complex clinic-based intervention for integrating anti-retro viral (ART) treatment into routine antenatal care in Zambia for post-partum women.

Part 3: study design features and their role in disambiguating study design labels
Because there are no reasons to believe that these individuals would have had systematically different characteristics to others, the exclusion of individuals was considered “as good as random” (i.e., a true natural experiment based on quasi-random assignment) [9]. Part 1 sets out designs currently used for health systems evaluations, illustrating their use through inclusion of different designs/analyses in a recent systematic review. Part 3 clarifies some of the ambiguities of study design labels using the proposed design feature framework. A category of alternative explanations for differences between scores such as events that happened between the pretest and posttest, unrelated to the study.
Explanatory Research – Types, Methods, Guide
Potential threats to internal validity are illustrated along with means of addressing their potentially biasing effects so that these effects can be minimized. In contrast to quasi-experiments, randomized experiments are often thought to be the gold standard when estimating the effects of treatment interventions. However, circumstances frequently arise where quasi-experiments can usefully supplement randomized experiments or when quasi-experiments can fruitfully be used in place of randomized experiments. Researchers need to appreciate the relative strengths and weaknesses of the various quasi-experiments so they can choose among pre-specified designs or craft their own unique quasi-experiments. Imagine, for example, that students in one school are given a pretest on their attitudes toward drugs, then are exposed to an antidrug program, and finally are given a posttest.
Qualitative Research Methods
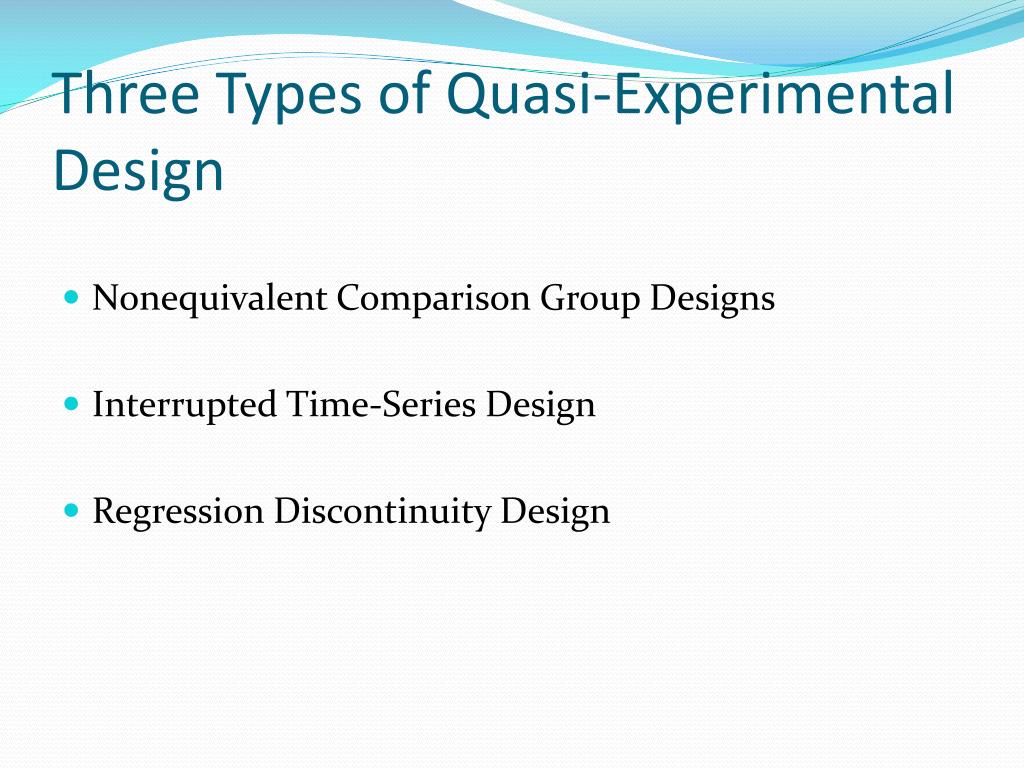
For example, if the pharmacy order-entry system involves an educational component, then people may apply the knowledge learned to nonintervention wards, thereby potentially masking the true effect of the intervention. When a design using randomized locations is employed successfully, the locations may be different in other respects (confounding variables), and this further complicates the analysis and interpretation. In a true experiment investigating the effects of a new medication on a specific condition, researchers would randomly assign participants to either the experimental group, which receives the medication, or the control group, which receives a placebo. In a quasi-experiment, researchers might instead compare patients who voluntarily choose to take the medication to those who do not, examining the differences in outcomes between the two groups. Quasi-experimental designs encompass various approaches, including nonequivalent group designs, interrupted time series designs, and natural experiments. Each design offers unique advantages and limitations, providing researchers with versatile tools to explore causal relationships in different contexts.
The statistical fact that an individual who scores extremely on a variable on one occasion will tend to score less extremely on the next occasion. When you wish to explain any complex data, it’s always advised to break it down into simpler visuals or stories. It is a platform that helps researchers and scientists to turn their data into easy-to-understand and dynamic stories, helping the audience understand the concepts better. We present a decision ‘map’ approach based on a Figure 5 to assist in considering decisions in selecting among QEDs and for which features you can pay particular attention to in the design [Figure 5 here]. In a genuine trial, you’d split half of the psych ward into treatment groups, With half getting the new psychotherapy therapy and the other half receiving standard depression treatment. Below are listed a few tools and online guides that can help you start your Quasi-experimental research.
RCTs can also involve random assignment of groups (e.g., clinics, worksites or communities) to intervention and control arms, but a large number of groups are required in order to realize the full benefits of randomization. Traditional RCTs strongly prioritize internal validity over external validity by employing strict eligibility criteria and rigorous data collection methods. QEDs test causal hypotheses but, in lieu of fully randomized assignment of the intervention, seek to define a comparison group or time period that reflects the counter-factual (i.e., outcomes if the intervention had not been implemented) (43). QEDs seek to identify a comparison group or time period that is as similar as possible to the treatment group or time period in terms of baseline (pre-intervention) characteristics. QEDs can include partial randomization such as in stepped wedge designs (SWD) when there is pre-determined (and non-random) stratification of sites, but the order in which sites within each strata receive the intervention is assigned randomly.
Types Of Quasi-Experimental Designs
For medical informatics interventions, it is often difficult to randomize the intervention to individual patients or to individual informatics users. So while this randomization is technically possible, it is underused and thus compromises the eventual strength of concluding that an informatics intervention resulted in an outcome. For example, randomly allowing only half of medical residents to use pharmacy order-entry software at a tertiary care hospital is a scenario that hospital administrators and informatics users may not agree to for numerous reasons. An experiment is a study in which the researcher manipulates the level of some independent variable and then measures the outcome. Many researchers consider experiments the "gold standard" against which all other research designs should be judged.
Or the principal might have assigned the “troublemakers” to Mr. Jones’s class because he is a stronger disciplinarian. Of course, the teachers’ styles, and even the classroom environments, might be very different and might cause different levels of achievement or motivation among the students. If at the end of the study there was a difference in the two classes’ knowledge of fractions, it might have been caused by the difference between the teaching methods—but it might have been caused by any of these confounding variables. Although quasi-experimental study designs are ubiquitous in the medical informatics literature, as evidenced by 34 studies in the past four years of the two informatics journals, little has been written about the benefits and limitations of the quasi-experimental approach.
A quasi-experimental study (also known as a non-randomized pre-post intervention) is a research design in which the independent variable is manipulated, but participants are not randomly assigned to conditions. In conclusion, quasi-experimental designs offer valuable opportunities for researchers to investigate cause-and-effect relationships in diverse fields, ranging from healthcare to social sciences. Despite their limitations, such as the risk of confounding bias and lower internal validity compared to true experiments, quasi-experimental methods provide a practical and ethical approach to studying complex phenomena. In a pretest-posttest design, the dependent variable is measured once before the treatment is implemented and once after it is implemented.
The health care evaluation community has historically been much more difficult to win around to the potential value of nonrandomized studies to evaluate interventions. We think that the checklist helps to explain why, that is, because designs used in health care evaluation do not often control for unobservables when the study features are examined carefully. However, to the extent that studies may be possible with features that promote the credibility of causal inference, health care evaluation researchers may be missing an opportunity to provide high-quality evidence. For example, a manufacturing company might measure its workers’ productivity each week for a year. In an interrupted time series-design, a time series like this one is “interrupted” by a treatment. In one classic example, the treatment was the reduction of the work shifts in a factory from 10 hours to 8 hours (Cook & Campbell, 1979)[5].
Common statistical tests used in quasi-experimental designs include t-tests, ANOVA, and regression analysis. The quasi-experimental design, similar to true experiments, is a research design that aims to identify the causal relationship between an independent and dependent variable. However, unlike true experiments, quasi-experimental studies utilize non-random criteria while assigning subjects to groups. In experimental research, researchers divide participants into an experimental group and a control group. In a true experiment, researchers determine which group each participant joins through a random assignment. Reflecting on the circumstances of nonrandomized evaluations of health care and health system interventions may provide some insights why these different groups have disagreed about the credibility of effects estimated in quasi-experimental studies.


















